Bromine Carbon Tetrachloride Formula . alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Write the symbol for each. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. Predict which forms an anion, which forms a cation, and the charges of each ion. aluminum and carbon react to form an ionic compound. The bromine reagent is in. The bromines add to opposite faces of the double bond. Philipp's answer is good, but structures a and b are both drawn in misleading ways. when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [ note 1].
from chemtube3d.com
alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Philipp's answer is good, but structures a and b are both drawn in misleading ways. aluminum and carbon react to form an ionic compound. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in. Write the symbol for each. carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. The bromines add to opposite faces of the double bond. when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [ note 1]. Predict which forms an anion, which forms a cation, and the charges of each ion.
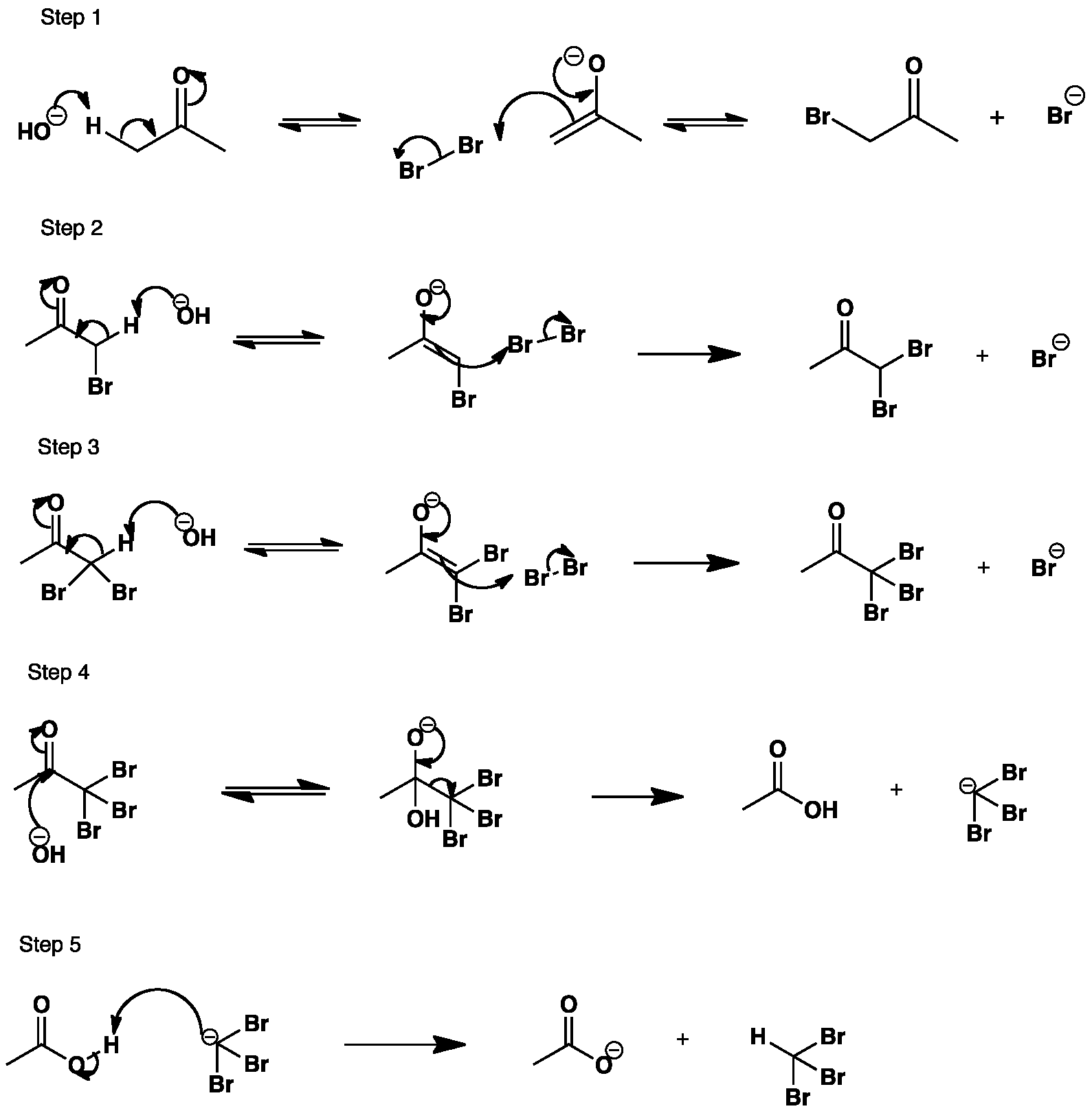
BaseCatalysed Bromination of Ketones Summary
Bromine Carbon Tetrachloride Formula carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. The bromine reagent is in. Write the symbol for each. carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [ note 1]. Philipp's answer is good, but structures a and b are both drawn in misleading ways. The bromines add to opposite faces of the double bond. Predict which forms an anion, which forms a cation, and the charges of each ion. alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. aluminum and carbon react to form an ionic compound.
From www.numerade.com
SOLVED organic compound with the molecular formula C8H6 decolorizes Bromine Carbon Tetrachloride Formula when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [ note 1]. carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. The bromine reagent is in. The bromines add to opposite faces of the double bond. Write the symbol for. Bromine Carbon Tetrachloride Formula.
From www.shutterstock.com
Vektor Stok Carbon Tetrachloride Ccl4 Structural Chemical Formula Bromine Carbon Tetrachloride Formula aluminum and carbon react to form an ionic compound. Philipp's answer is good, but structures a and b are both drawn in misleading ways. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. alkenes react in the cold with pure liquid. Bromine Carbon Tetrachloride Formula.
From www.numerade.com
SOLVED Problem 4 What is the volume percent (v/v) of bromine in a Bromine Carbon Tetrachloride Formula alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine reagent is in. Philipp's answer is good, but structures a and b are both drawn in misleading ways. when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2). Bromine Carbon Tetrachloride Formula.
From www.chemtex.net
Carbon Tetrachloride Liquid Manufacturer, Exporter from Kolkata Bromine Carbon Tetrachloride Formula The bromines add to opposite faces of the double bond. The bromine reagent is in. Write the symbol for each. when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [ note 1]. the reaction between c=c double bond and bromine (br 2) can be used as. Bromine Carbon Tetrachloride Formula.
From www.teachoo.com
Unsaturated hydrocarbons contain multiple bonds between 2 carbon atoms Bromine Carbon Tetrachloride Formula The bromine reagent is in. aluminum and carbon react to form an ionic compound. Write the symbol for each. Philipp's answer is good, but structures a and b are both drawn in misleading ways. alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromines add. Bromine Carbon Tetrachloride Formula.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Bromine Carbon Tetrachloride Formula when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [ note 1]. The bromine reagent is in. Write the symbol for each. Predict which forms an anion, which forms a cation, and the charges of each ion. The bromines add to opposite faces of the double bond.. Bromine Carbon Tetrachloride Formula.
From www.pinterest.com
Carbon tetrachloride CCl₄ Molecular Geometry Hybridization Bromine Carbon Tetrachloride Formula when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [ note 1]. carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. the reaction between c=c double bond and bromine (br 2) can be used as a test for the. Bromine Carbon Tetrachloride Formula.
From www.chegg.com
Solved Complete The Mechanism And The Products For The Re... Bromine Carbon Tetrachloride Formula Predict which forms an anion, which forms a cation, and the charges of each ion. alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Write the symbol for each. the reaction between c=c double bond and bromine (br 2) can be used as a test for. Bromine Carbon Tetrachloride Formula.
From www.chegg.com
Solved Determine the likely structure for a compound A Bromine Carbon Tetrachloride Formula Predict which forms an anion, which forms a cation, and the charges of each ion. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in. carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic. Bromine Carbon Tetrachloride Formula.
From brainmass.com
Determining Molecules from Spectral Diagrams Bromine Carbon Tetrachloride Formula alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine reagent is in. when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [ note 1]. carbon tetrachloride, a colourless, dense, highly toxic,. Bromine Carbon Tetrachloride Formula.
From www.animalia-life.club
Ch3ch(oh)ch3 Structural Formula Bromine Carbon Tetrachloride Formula The bromine reagent is in. alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. Predict which forms an anion, which forms a cation, and the charges of each ion. aluminum. Bromine Carbon Tetrachloride Formula.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Bromine Carbon Tetrachloride Formula Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromines add to opposite faces of the double bond. Philipp's answer is good,. Bromine Carbon Tetrachloride Formula.
From www.dreamstime.com
Bromine, Br, Periodic Table Element Stock Illustration Illustration Bromine Carbon Tetrachloride Formula The bromines add to opposite faces of the double bond. carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. Predict which forms an anion, which forms a cation, and the charges of each ion. when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in. Bromine Carbon Tetrachloride Formula.
From www.vecteezy.com
Bromine symbol. Chemical element of the periodic table. Vector Bromine Carbon Tetrachloride Formula aluminum and carbon react to form an ionic compound. Write the symbol for each. alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromines add to opposite faces of the double bond. carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a. Bromine Carbon Tetrachloride Formula.
From www.numerade.com
SOLVED 5. Write a chemical equation for the reaction of acetylene with Bromine Carbon Tetrachloride Formula when alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [ note 1]. Predict which forms an anion, which forms a cation, and the charges of each ion. Philipp's answer is good, but structures a and b are both drawn in misleading ways. The bromine reagent is in.. Bromine Carbon Tetrachloride Formula.
From www.meritnation.com
Draw the electron dot structure of "Carbon Tetrachloride" 9583799 Bromine Carbon Tetrachloride Formula carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. Philipp's answer is good, but structures a and b are both drawn in misleading ways. The bromines add to opposite faces of the double bond. The bromine reagent is in. aluminum and carbon react to form an ionic compound. when alkenes (. Bromine Carbon Tetrachloride Formula.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Carbon Tetrachloride Formula carbon tetrachloride, a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and. The bromine reagent is in. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. when alkenes ( also known as olefins) are treated with bromine (br. Bromine Carbon Tetrachloride Formula.
From fphoto.photoshelter.com
science chemistry reaction bromine carbon tetrachloride Fundamental Bromine Carbon Tetrachloride Formula Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each. alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromines add to opposite faces of the double bond. when alkenes ( also known as olefins). Bromine Carbon Tetrachloride Formula.